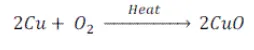Q.1 Why should a magnesium ribbon be cleaned before burning in air?
Sol.
Magnesium forms magnesium oxide when reacts with oxygen present in air. On the magnesium ribbon a stable layer of magnesium oxide is formed when it is stored, which prevents the further reaction of magnesium ribbon. Hence, to clean up the layer of magnesium oxide, magnesium ribbon is cleaned before burning.
Q.2 Write the balanced equation for the following chemical reactions.
(i) Hydrogen + Chlorine → Hydrogen chloride
(ii) Barium chloride + Aluminium sulphate → Barium sulphate + Aluminium chloride
(iii) Sodium + Water → Sodium hydroxide + Hydrogen
Sol.
(i) H2 + Cl2 → 2HCl
(ii) 3 BaCl2 + Al2(SO4)3 → BaSO4 + 2 AlCl3
(iii) 2Na + 2H2O → 2NaOH + H2↑
Q.3 Write a balanced chemical equation with state symbols for the following reactions.
(i) Solutions of barium chloride and sodium sulphate in water react to give insoluble barium sulphate and the solution of sodium chloride.
(ii) Sodium hydroxide solution (in water) reacts with hydrochloric acid solution (in water) to produce sodium chloride solution and water.
Sol.
(i) BaCl2 (aq) + Na2SO4 (aq) → BaSO4(s) + 2NaCl (aq)
(ii) NaOH (aq) + HCl(aq) → NaCl(aq) + H2O(l)
Page Number 10
Q.1 A solution of a substance ‘X’ is used for white washing.
(i) Name the substance ‘X’ and write its formula.
(ii) Write the reaction of the substance ‘X’ named in (i) above with water.
Sol.
i) The substance ‘X’ which is used in whitewashing is quick lime or Calcium Oxide and its formula is CaO.
ii) CaO + H2O → Ca(OH)2
Q.2 Why is the amount of gas collected in one of the test tubes in Activity 1.7 double of the amount collected in the other? Name this gas.
Sol.
(Hydrolysis of water has been performed in the activity 1.7 in the text book.)
When electric current is passed through water it decomposes into its constituent components, i.e. hydrogen and oxygen gases. During electrolysis of water hydrogen gas collected in one test tube and has double amount than oxygen collected in another test tube. This is because a water molecule is formed by the combination of hydrogen and oxygen in the ratio of 2:1.
The reaction involved here is:
2H2O(l) → 2H2(g) + O2(g)
Page Number 13
Q.1 Why does the colour of copper sulphate solution change when an iron nail is dipped in it?
Sol.
The colour of copper sulphate solution is blue. When an iron nail is dipped in the solution of copper sulphate, it forms ferrous sulphate solution and copper metal by the displacement of copper from the solution.
The colour of ferrous sulphate is green. That’s why the solution becomes green.
The reaction involved here is:
Fe (s) + CuSO4 (aq) → FeSO4 (aq) + Cu (s)
Q.2 Give an example of a double displacement reaction other than the one given in Activity 1.10.
Sol.
Pb(NO3)2+2KI→PbI2+2KNO3
When lead nitrate reacts with potassium iodide, potassium nitrate and lead iodide is formed. In this reaction the both the reactants exchanges their irons after reaction. This type of reaction is known as double displacement reaction.
Q.3 Identify the substances that are oxidised and the substances that are reduced in the following reactions.
(i) 4Na(s) + O2(g) → 2Na2O(s)
(ii) CuO (s) + H2(g) → Cu (s) + H2O(l)Sol.
(i) In this reaction sodium is combined with oxygen, i.e. gains oxygen and hence oxidized. Consequently oxygen is reduced.
(ii) In this reaction Hydrogen gains oxygen and forms water, hence it is oxidised. On the other hand copper loses oxygen and hence, reduced.
Back Exercise Questions
Q.1 Which of the statements about the reaction below are incorrect?
2PbO(s) + C(s) → 2Pb (s) + CO2(g)
(a) Lead is getting reduced.
(b) Carbon dioxide is getting oxidized.
(c) Carbon is getting oxidized.
(d) Lead oxide is getting reduced.
(i) (a) and (b)
(ii) (a) and (c)
(iii) (a), (b) and (c)
(iv) all
Sol.
(i) (a) and (b)
Q.2 Fe2O3 + 2Al → Al2O3 + 2Fe
The above reaction is an example of a
(a) combination reaction.
(b) double displacement reaction.
(c) decomposition reaction.
(d) displacement reaction.
Sol.
(d) displacement reaction.
Q.3 What happens when dilute hydrochloric acid is added to iron fillings? Tick the correct answer.
(a) Hydrogen gas and iron chloride are produced.
(b) Chlorine gas and iron hydroxide are produced.
(c) No reaction takes place.
(d) Iron salt and water are produced.
Sol.
(a) Hydrogen gas and iron chloride are produced.
Q.4 What is a balanced chemical equation? Why should chemical equations be balanced?
Sol.When the number of atoms of reactants is equal to the number of atoms of the products, the reaction is called a balanced chemical equation. According to the Law of conservation of mass, total mass of the elements present in the reactants must be equal to the total mass of the elements present in products. That’s why a chemical equations should be balanced always.
Q.5 Translate the following statements into chemical equations and then balance them.
(a) Hydrogen gas combines with nitrogen to form ammonia.
(b) Hydrogen sulphide gas burns in air to give water and sulphur dioxide.
(c) Barium chloride reacts with aluminium sulphate to give aluminium chloride and a precipitate of barium sulphate.
(d) Potassium metal reacts with water to give potassium hydroxide and hydrogen gas.
Sol.
(a) 3H2 (g) + N2 (g) → 2NH3 (g)
(b) H2S (g) + 3O2 (g) → SO2 (g) + 2H2O(l)(c) 3BaCl2 (aq) + Al2(SO4)3 (aq) → 2AlCl3 (aq) + 3BaSO4 ↓(s)(d) 2K (s) + 2H2O (l) → 2KOH (aq) + H2 (g)
Q.6 Balance the following chemical equations:
(a) HNO3 + Ca (OH)2 → Ca (NO3)2 + H2O
(b) NaOH + H2SO4 → Na2SO4 + H2O
(c) NaCl + AgNO3 → AgCl + NaNO3
(d) BaCl2 + H2SO4 → BaSO4 + HClSol.
(a) 2HNO3 + Ca(OH)2 → Ca(NO3)2 + 2H2O
(b) 2NaOH + H2SO4 → Na2SO4 + 2H2O(c) NaCl + AgNO3 → AgCl + NaNO3(d) BaCl2 + H2SO4 → BaSO4 + 2HCl
Q.7 Write the balanced chemical equations for the following reactions.
(a) Calcium hydroxide + Carbon dioxide → Calcium carbonate + Water
(b) Zinc + Silver nitrate → Zinc nitrate + Silver
(c) Aluminium + Copper chloride → Aluminium chloride + Copper
(d) Barium chloride + Potassium sulphate → Barium sulphate + Potassium chloride
Sol.
(a) Ca (OH)2 + CO2 → CaCO3 + H2O
(b) Zn + 2AgNO3 → Zn(NO3)2 + 2 Ag(c) 2Al + 3 CuCl2 → 2AlCl3 + 3 Cu(d) BaCl2 + K2SO4 → BaSO4 + 2KCl
Q.8 Write the balanced chemical equation for the following and identify the type of reaction in each case.
(a) Potassium bromide(aq) + Barium iodide(aq) → Potassium iodide(aq) + Barium bromide(s)
(b) Zinc carbonate(s) → Zinc oxide(s) + Carbon dioxide(g)
(c) Hydrogen(g) + Chlorine(g) → Hydrogen chloride(g)
(d) Magnesium(s) + Hydrochloric acid(aq) → Magnesium chloride(aq) + Hydrogen(g)
Sol.
(a) 2KBr (aq) + Bal2(aq) → 2Kl(aq) + BaBr2(s)
Type : Double displacement reaction
(b) ZnCO3 (s) → ZnO (s) + CO2 (g)
Type : Decomposition reaction
(c) H2 (g) + Cl2 (g) → 2HCl(g)
Type : Combination reaction
(d) Mg (s) + 2HCl (aq) → MgCl2 (aq) + H2 (g)
Type : Displacement reaction
Q.9 What does one mean by exothermic and endothermic reactions? Give examples.
Sol.
Q.17 A shiny brown coloured element ‘X’ on heating in air becomes black in colour. Name the element ‘X’ and the black coloured compound formed.
Sol.
Copper is a brown coloured shiny element, when heated in air, it becomes black in colour.
2Cu+O2→2CuO
Hence, the element ‘X’ is copper and black coloured compound formed is copper oxide.
Q.18 Why do we apply paint on iron articles?
Sol.
When articles made of iron come in contact with the moisture present in air, it forms iron oxide, which is known as rust. Hence, to prevent an iron article to come in contact with moisture present in air paint is applied. Paint prevents the iron to get rusted.
Q.19 Oil and fat containing food items are flushed with nitrogen. Why?
Sol.
When fats and oils are oxidised, they become rancid and their smell and taste change. Hence, food items containing oil and fat are flushed with nitrogen which prevents them to get oxidized and becoming rancid.
Q.20 Explain the following terms with one example each
(a) Corrosion
(b) Rancidity
Sol. (a) Corrosion – Metals react with oxygen which is present in the atmospheric moisture. This leads to the formation of metal oxides. In due course of time, the metal keeps on changing into its oxide and finally the whole metal is lost due to oxidation. This process is called corrosion.
Example: Iron articles; like iron gates or bridges tend to rust because of oxidation by atmospheric moisture. We know that rust is nothing but iron oxide. Conversion of iron into rust leads to corrosion of the iron articles. Due to this, the iron articles weaken and finally wither away.
(b) Rancidity – When fats and oils are oxidised, their smell and taste change. This process is called rancidity. Oily food often become rancid and start giving out obnoxious smell. The taste also becomes bad. Such oily food is not fit for eating.
Example: When packets of potato chips or other oily snacks are kept open for a long time; their taste and smell become bad. The oily food is no longer safe to eat.